Classify the following processes as exothermic or endothermic. Classify the following processes as exothermic or endothermic.
Solved Part A Classify Each Process As An Endothermic Or Chegg Com
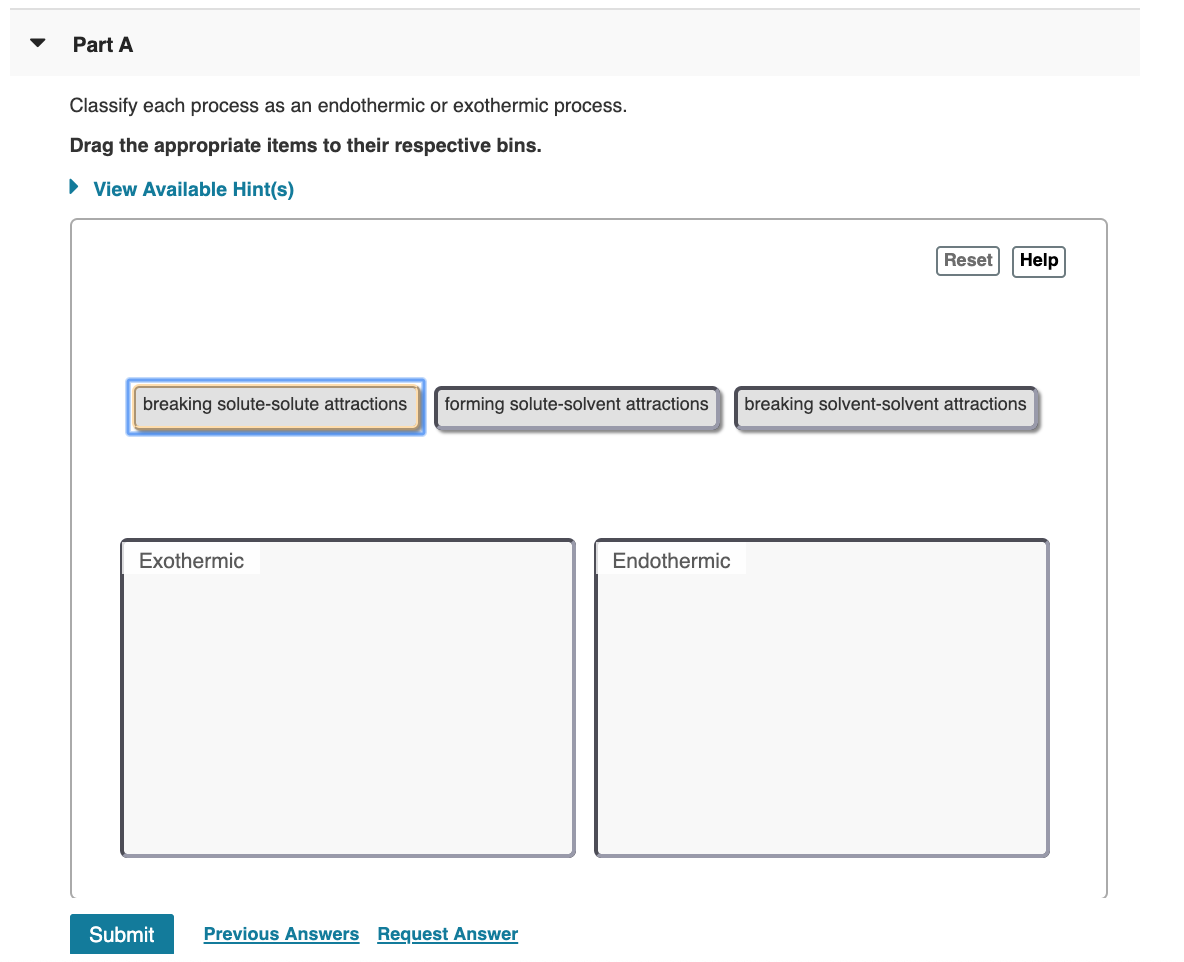
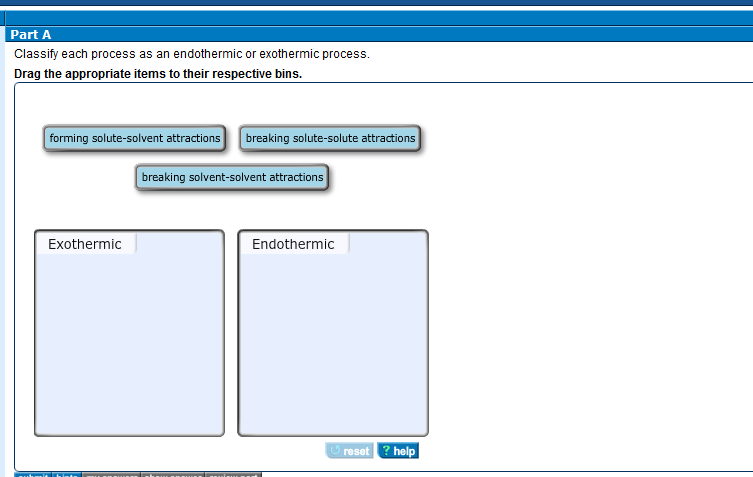
1 forming solute-solvent attractions 2 breaking solute-solute attractions 3 breaking solvent-solvent attractions.
. An exothermic is a reaction in which the surroundings absorb energy from the system energy usually in the form of heat. Chemistry questions and answers. E a person growing.
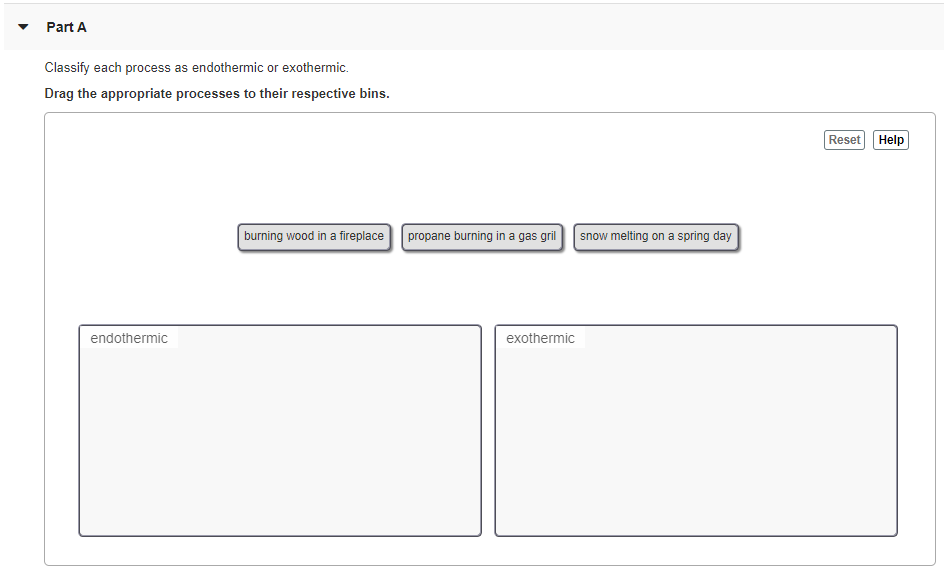
Gasoline burning in an engineb. A Your hand gets cold when you touch ice. Classify each process as exothermic or endothermic.
Liquefaction liquid from solid or gas c. An endothermic is a reaction in which the system absorbs energy from the surroundings in the form of heat. Classify each process as.
B The ice gets warmer when you touch it. A chemical reaction that feels cold. The separation of solute particles ΔH1 and the separation of solvent molecules ΔH2 are endothermic process whereas the formation of the attractive interaction ΔH3 is an exothermic process.
F wood being chopped. Forming solute-solvent attractions b. Exothermic - as a result of the chemical change thermal energy is released into the surroundings.
Breaking solvent-solvent attractions c. Science Chemistry QA Library Classify each process as endothermic or exothermic. Vaporization liquid to gas.
C digestion of food. HERE THE ANSWERS. Condensation gas to liquid b.
A chemical reaction that feels warm. Solution for Classify each process as endothermic or exothermic. Endothermic - thermal energy transformed into chemical energy in the products.
E Ice cream melts. Gasoline burning in an engine b. D a person running.
Here ΔH is the change in energy associated with each process. Find step-by-step Chemistry solutions and your answer to the following textbook question. Classify each process as an endothermic or exothermicprocess.
Classify each process as an endothermic or exothermic process. Exo Endo 25 Heat energy flows into the ice. What is the sign of delta H for each process.
Breaking solute-solute attractions Breaking solvent-solvent attractions. Gasoline burning in an engineb. A freezing of water b boiling of water c digestion of food.
Water boiling in a pot Provide at least two additional examples of exothermic processes and two additional examples of endothermic processes. 1 Classify each of the following processes as endothermic or exothermic. Endothermic Exothermic Answer Bank ice melting water condensing on a surface a car using gasoline baking a cake the chemical reaction inside an instant cold pack A tv W MacBook Air.
Freezing liquid to solid b. Steam condensing on a mirrorc. C Water boils in a kettle being heated on a stove.
A dry ice subliming 0041. Classify each process as endothermic or exothermic. G heating with a furnace.
Boiling liquid to gas 2 Classify each of the following processes as endothermic or exothermic. F wood being chopped. Ice melting Endothermic A sparkler burning Exothermic - Acetone evaporating from skin.
Steam condensing on a mirror c. Forming solute-solvent attractions Endothermic. Water boiling in a potProvide at least two additional examples of exothermic processes and two additional examples of endothermic.
The system is underlined in each example. E a person growing. D a person running.
G heating with a furnace. Classify the processes as endothermic or exothermic. What is the sign of ΔH for each process.
A freezing of water. What is thesign of H for each process. Classify each process as exothermic or endothermic Explain.
Sublimation solid to gas c. B boiling of water. D Water vapor condenses on a cold pipe.
Solved Classify Each Process As An Endothermic Or Exothermic Chegg Com

Difference Between Endothermic And Exothermic Reactions Chemistry

Solved Part A Classify Each Process As Endothermic Or Chegg Com

Exothermic And Endothermic Processes Chemistry For Non Majors
0 Comments